Chưa có sản phẩm trong giỏ hàng.
Tin tức, Công nghệ & sản phẩm mới, Hoạt động công ty, Khoa học và đời sống
Liquid Chromatograph Mass Spectrometer LC/MS
 Liquid chromatograph mass spectrometer (LC/MS) is, as its name suggests, a device that is a combination of a liquid chromatograph (LC) and a mass spectrometer (MS). The LC/MS is used in a wide range of areas.
Liquid chromatograph mass spectrometer (LC/MS) is, as its name suggests, a device that is a combination of a liquid chromatograph (LC) and a mass spectrometer (MS). The LC/MS is used in a wide range of areas.
A gas chromatograph mass spectrometer (GC/MS) is an analytical instrument for volatile substances. In comparison, the LC/MS is being used as an instrument applicable for a wide range of materials, from volatile substances to refractory substances.
Recently, the trend in chromatograph analysis has been a shift from gas chromatography (GC) to liquid chromatography (LC). The reason is that for volatile substances, a GC can be used, but with refractory substances, the GC cannot be used unless some chemical treatment, such as derivatization, is conducted to make the refractory substance volatile. On the other hand, with LC, the derivatization is not needed, and the target sample area can be much wider than the one used with GC. However, in case of an LC, there are some aspects of the detector that create difficulties, so selection must be made to correspond to the sample and characteristics. But using an MS as a detector offers the potential for greater versatility and improved identification accuracy. In addition, the appearance of interfaces to effectively connect LC and MS, as well as performance improvements have led to a rapidly expanding demand for LC/MS.
LC/MS Interfaces
Since the 1970’s, interfaces for LC/MS have been a subject of intense study, and a variety of methods have been proposed and implemented. Among them are, the belt method, in which the mobile phase from LC is dropped onto a belt, heated and then measured using the electron ionization (EI) method; the spray method, in which the sample is sprayed as a gas aerosol and ionized using the EI method; and a thermo spray method. In late 1980’s, the atmospheric pressure ionization method (API) appeared, and has become widely used. It is the standard LC/MS interface today. While LC can use liquid at atmosphere pressure as the mobile phase, MS requires a high vacuum. The interface which successfully combines the conflicting natures of these is the atmospheric pressure ionization method ”API” method. There are two kinds of ionization method for API, one is Electrospray Ionization (ESI) method, and the other is the Atmospheric Pressure Chemical Ionization (APCI) method.
1) ESI Method
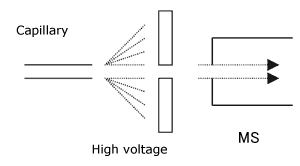
This is an ionization method in which the mobile phase solution is introduced into a capillary and a high voltage is applied between this capillary and the opposing electrode.
The feature of this method is that the ionization energy is the smallest among the existing ionization methods. As a result, this is the only ionization method which can obtain the molecular weight information of not only biological polymer samples, but also coordinate bond compounds such as coordination complexes. In addition, when this method is applied to protein, peptide, or nucleic acid, it enables observation as a multivalent ion.
For example, when a compound with a molecular weight of 10,000 is observed as +10-valence ion, the m/z value (mass/charge ratio) of the obtained mass spectrum turns out to be 1,001. The spectrum of a multivalent ion can be obtained as a characteristic spectrum with a distribution, and allows a precise calculation of the molecular weight using a computer.
2) APCI Method
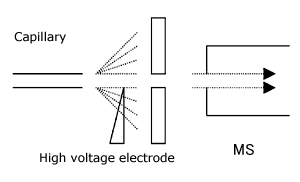
As can be inferred from the name “atmospheric pressure chemical ionization”, this is a method utilizing the mobile phase solvent to fill a similar role as the reagent gas in chemical ionization to measure the molecular ions with added protons and ions with the mobile phase solvent added. The ionization is performed by introducing an aerosol of the sample solution into a corona discharge area. The target samples are compounds with a lower polarity than those suitable for ESI.
In addition, to conduct chemical ionization, the mobile phase solvent concentration must be high, so a flow rate of 1 ml or more per minute is used.
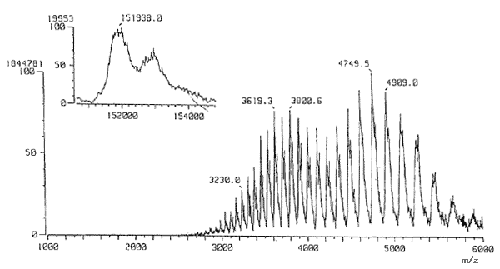
An analysis example using the APCI method is shown below. The target sample is green tea. Green tea has a lot of catechins, which are famous for a wide range of effects, including antibacterial effects. The measurement results of 20μl of ordinary green tea injected into an LC are as shown below.
The measurement results clearly show that green tea contains a lot of amino acids, catechins, caffeine, and rutin.
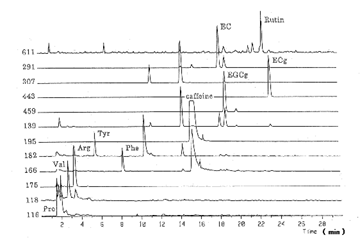
As this illustrates, LC/MS can analyze the components of a wide range of samples from biological polymers to green tea, easily and with high sensitivity.
Source: Jeol.com
