Introduction
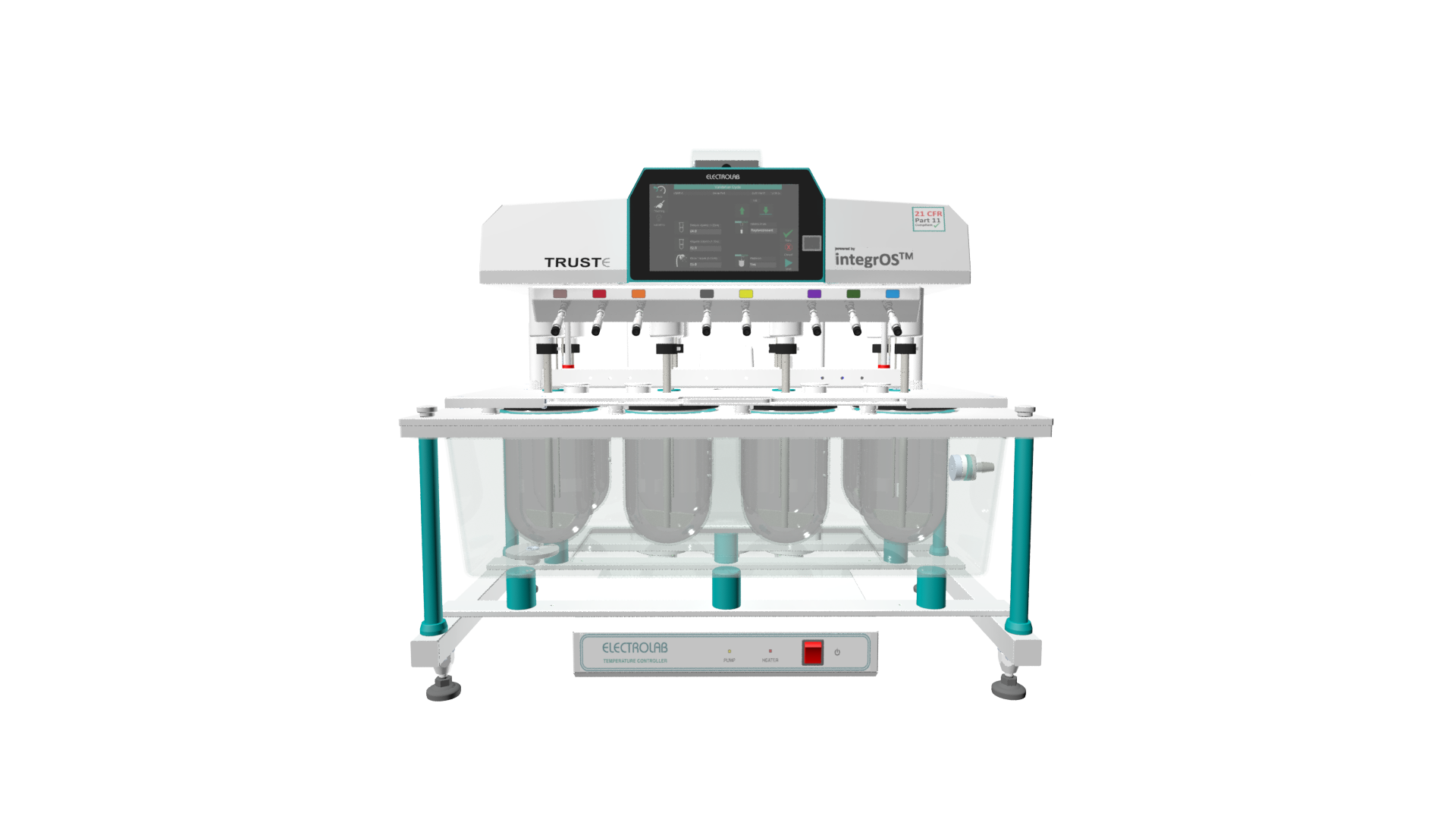
The TrustE-08 Dissolution Tester Manual Syringe Sampling is an 8-station instrument for USP-compliant dissolution testing. It combines advanced regulatory features with a manual sampling workflow. This tester is controlled by a 7-inch touch screen and features a motorized lift. The TrustE-08 is designed for labs that require data integrity and user control.
Product Overview
This 8-station dissolution tester is built for pharmaceutical labs that need full 21 CFR Part 11 compliance in a manual sampling setup. The system runs on IntegrOS software, providing features like fingerprint authentication, audit trails, and e-signatures. Its unique TimeAction™ function allows for programmed media changeovers, which is ideal for testing extended-release products. The TrustE-08 Dissolution Tester Manual Syringe Sampling provides a secure, traceable, and user-friendly platform for both quality control and research and development activities.
Key Features
- 8-Station Capacity: Allows for testing of up to 8 samples simultaneously.
- 21 CFR Part 11 Compliant: Features IntegrOS software with fingerprint login, audit trails, and e-signatures.
- 7-inch Touch Screen: Provides an intuitive interface for programming and monitoring tests.
- USP Compliance: Supports methods for USP Apparatus 1, 2, 5, and 6.
- Motorized Lift Mechanism: Ensures smooth, vibration-free movement of the stirrer head.
- TimeAction™ Function: Enables programmed media changeovers for complex dissolution profiles.
Technical Specifications
| Model | TrustE-08 Dissolution Tester Manual Syringe Sampling |
| No. of stations | 8 |
| Speed range | 20 RPM to 300 RPM |
| Temperature range | 20°C to 40°C |
| Display | 7″ TFT touch screen |
| Vessel capacity | 150ml, 250ml, 1L |
| Dimensions (W x L x H) | 517 mm x 712 mm x 690 mm |
Applications
- Quality control testing of solid dosage forms like tablets and capsules.
- Dissolution profiling of immediate and extended-release drugs.
- Methods requiring USP 1 (Basket), 2 (Paddle), 5 (Paddle over Disk), and 6 (Rotating Cylinder).
- Laboratories requiring 21 CFR Part 11 data integrity with a manual sampling process.
Pricing
Please contact us for consultation and detailed quotation.
Frequently Asked Questions (FAQ)
1. What is the TrustE-08 Dissolution Tester Manual Syringe Sampling used for?
It is used to perform USP-compliant dissolution tests for up to 8 samples, combining advanced regulatory compliance features with a manual sampling workflow.
2. Is this model fully automated?
No, this model is designed for manual syringe sampling, giving the operator direct control over the sampling process.
3. Does the TrustE-08 meet FDA 21 CFR Part 11 requirements?
Yes, through its IntegrOS software, it offers full 21 CFR Part 11 compliance features, including fingerprint login, audit trails, and e-signatures.
4. What USP methods are supported by this tester?
It supports USP Apparatus 1 (Basket), 2 (Paddle), 5 (Paddle over Disk), and 6 (Rotating Cylinder).
5. What is the TimeAction™ function on the tester?
This function allows for programmed, automatic media changes, which is useful for mimicking the changing pH conditions of the GI tract for extended-release drugs.
Conclusion
The TrustE-08 Dissolution Tester Manual Syringe Sampling offers a compliant and user-friendly solution for manual dissolution testing. Its 8-station capacity and full 21 CFR Part 11 features make it a reliable choice for modern pharmaceutical labs. Contact us for more information.










Reviews
There are no reviews yet.