Introduction
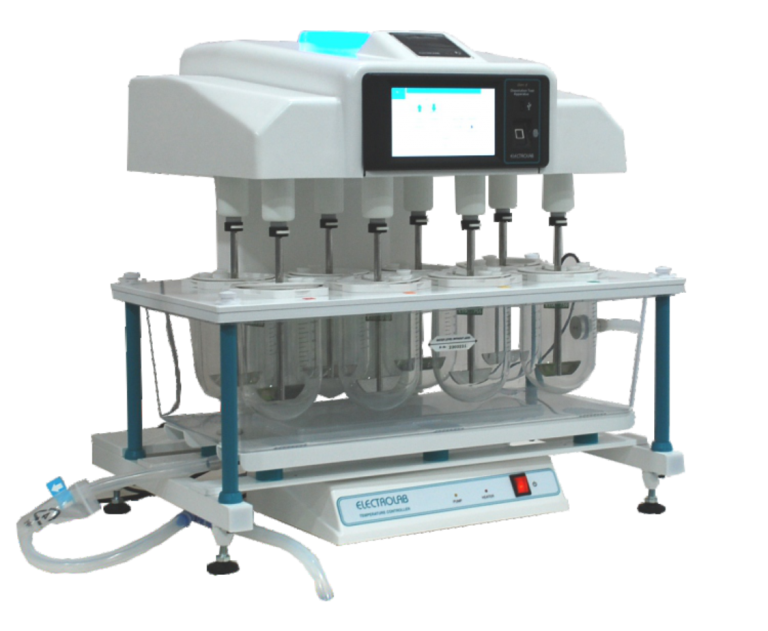
Dissolution Tester Trust E-14 Gen 2 (Basic) measures the rate at which solid dosage forms dissolve in a liquid medium. This instrument ensures that drug products release their active ingredients correctly. It features 14 test stations and complies with major pharmacopeias.
Product Overview
This apparatus is an essential tool for pharmaceutical quality control (QC) laboratories. It simulates in-vivo conditions to determine a drug’s performance. By providing accurate dissolution data, the tester helps ensure product safety and efficacy. The design of the Trust E-14 Gen 2 (Basic) focuses on reliability and compliance with international pharmacopeial regulations, making it a staple for routine testing.
Key Features
- Station Capacity: 14 test stations allow for high-throughput analysis.
- Compliance: Fully compliant with USP, IP, Ph. Eur., and JP specifications.
- Interface: A 7-inch color TFT touch screen for easy operation.
- TimeAction™ Function: Allows media changeover and infinity test runs, ideal for sustained-release products.
- Stirrer Positioning: Automatic stirrer height positioning for USP 1, 2, 5, and 6 test methods.
- Security: Multi-level security with passwords for 250 users and biometric login.
- Data Storage: Stores up to 1000 product methods and 50,000 test reports.
Technical Specifications
| Model | TrustE-14 Basic (Gen-2) |
| Stations | 14 |
| Speed Range | 20 to 300 RPM (±0.5 RPM accuracy) |
| Temperature Range | 20°C to 40°C (±0.1°C accuracy) |
| Display | 7-inch TFT touch screen |
| Connectivity | Ethernet, RS-232, RS-485 |
| Dimensions (L x W x H) | 1150 x 521 x 685 mm |
| Weight | 126 Kg |
Applications
- Quality control testing of tablets and capsules.
- Research and development of new drug formulations.
- Testing of sustained and controlled-release drug products.
- Compliance testing according to pharmacopeial standards.
- Stability analysis of pharmaceutical products.
Pricing
Please contact us for consultation and detailed quotation.
FAQ
1. What is the Dissolution Tester Trust E-14 Gen 2 (Basic) used for?
It is used to measure the rate and extent of dissolution for active ingredients from solid dosage forms like tablets and capsules.
2. How many test stations does it have?
This model features 14 stations, enabling simultaneous testing of multiple samples for increased efficiency.
3. Does the Dissolution Tester Trust E-14 Gen 2 (Basic) comply with USP?
Yes, it is fully compliant with United States Pharmacopeia (USP) requirements, as well as Indian Pharmacopoeia (IP) and European Pharmacopoeia (Ph. Eur.).
4. What is the TimeAction™ function?
It is a feature that facilitates media changes and long-duration tests, which is particularly useful for extended-release formulations.
5. Can the device store test data?
Yes, it can store up to 50,000 test reports and 1000 product methods, along with a detailed audit trail for data integrity.
Conclusion
The Dissolution Tester Trust E-14 Gen 2 (Basic) is an efficient and compliant solution for dissolution testing in the pharmaceutical industry. This instrument provides reliable results for quality control and product development. Contact us for more information.










Reviews
There are no reviews yet.