Dissolved oxygen meter EzDO with Ezintegrity: 21 CFR Part 11 compliance for the pharmaceutical industry
In highly regulated industries like pharmaceuticals, measuring dissolved oxygen (DO) requires not only accuracy but also absolute data integrity and security. The EzDO Dissolved Oxygen Meter with Ezintegrity software by Electrolab is engineered to be the perfect solution, combining reliable measurement performance with full compliance with 21 CFR Part 11 regulations.
Why is EzDO with Ezintegrity the mandatory choice for regulated labs?
This instrument is equipped with leading technologies to meet the most stringent requirements for measurement and data management.
Comprehensive 21 CFR Part 11 compliance
This is the core feature that makes the EzDO an indispensable tool in a GxP environment. The integrated Ezintegrity software provides a full suite of security features, including multi-level user management, an unalterable audit trail, and electronic signatures, ensuring all data is secure and legally valid.
Accurate and reliable measurement
With a high resolution of 0.001 ppm and an accuracy of ±2% of full scale, the EzDO provides measurement results you can trust. The Automatic Temperature Compensation (ATC) feature eliminates errors caused by temperature, ensuring consistent results under various conditions.
Modern touch interface and smart operation
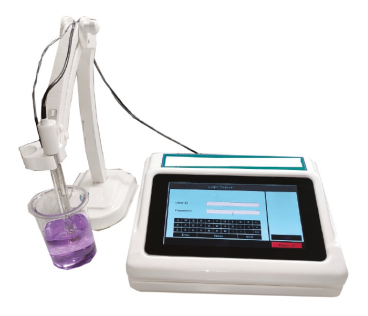
The 7-inch color TFT touch screen makes operation incredibly simple. The user-friendly graphical interface and built-in instructions allow users to easily perform calibrations, measurements, and data management without complex training.
Comprehensive data management and connectivity
With a large memory for 1000 reports, direct connectivity to printers via USB, network, or Wi-Fi, and data export to a USB drive, the EzDO with Ezintegrity offers maximum flexibility in managing and archiving records in accordance with GLP principles.
Detailed technical specifications
Model: EzDO with Ezintegrity
Compliance: 21 CFR Part 11
Parameter: Dissolved Oxygen (DO)
DO Range: 0 ppm to 20 ppm
DO Resolution: 0.001 ppm
DO Accuracy: ±2% FS (Full Scale)
Display: 7-inch TFT touch screen
Storage: 1000 reports
Connectivity: USB (printer, drive), Wi-Fi, Network
Frequently asked questions (FAQ)
1. What is the main difference between this model and the standard EzDO?
The core difference is the software. This model is integrated with Ezintegrity software, making it fully compliant with 21 CFR Part 11 regulations for electronic records and signatures. The standard EzDO is GLP compliant but lacks advanced data security features like an audit trail.
2. Why is 21 CFR Part 11 compliance important for a DO meter?
In pharmaceutical manufacturing, especially in cell culture or fermentation processes, dissolved oxygen concentration is a Critical Process Parameter (CPP). Ensuring that measurement data is accurate, secure, and unalterable is a mandatory requirement from regulatory agencies.
3. What is an “Audit Trail”?
An Audit Trail is a secure, computer-generated, time-stamped electronic record that independently records all actions related to the creation, modification, or deletion of a record. This trail cannot be altered, which helps ensure data integrity.
4. Can I connect this instrument to my lab’s LIMS?
Yes, with its network connectivity (Wi-Fi/LAN), data from the EzDO with Ezintegrity can be transferred to a computer and integrated into your Laboratory Information Management System (LIMS).
5. Do I need a computer to print my results?
No. One of the key advantages of this meter is its ability to connect directly to a printer via a USB port or over the network, allowing you to print GLP/21 CFR Part 11 compliant reports on the spot.










Reviews
There are no reviews yet.