Measurements can be made on almost any sample, but most often is used for soft matter and nanostructured materials. SAXS is a primary characterization tool for polymers, surfactants, colloids, proteins, porous media, nanoparticles and nanocomposites.
SAXS can be performed on samples in a dynamic or controlled environment, such as temperature, stress, humidity, pressure, concentration, in order to understand the link between structure and function or response.
Which information does SAXS give?
SAXS provides unequivocal determination of nanoscale structural and morphological information in any almost exhaustive range of materials, such as polymers, surfactants, colloids and proteins.
More specifically, the size domains measured by SAXS are continuously probed between roughly 1 nm to hundreds of nanometers, and can even extend to microns with Ultra-SAXS.
Some examples where SAXS is the unique measurement tool are:
- the structure determination of lamellar phase dimensions in Polyolefins
- identification of phases in block copolymers, surfactants and molecular objects, including quasi-crystalline and Frank-Kasper phases
- size and shape of micelles used in soaps, detergents and personal care products
- unambiguous determination of size in colloids used as additives to food, drugs and personal care products.
- nanoparticle size distribution
In structural biology, SAXS has long been used to provide shape analysis of proteins in solution, DNA & RNA assemblies, virus dimensions and shape, and combinations of all these – information not available by the standard tools of protein crystallography. Protein and antibody dynamics can be inferred by comparing the SAXS to structural models. Furthermore, protein stability can be studied under changing conditions.
Select an application of SAXS below to learn more:
How does SAXS work?
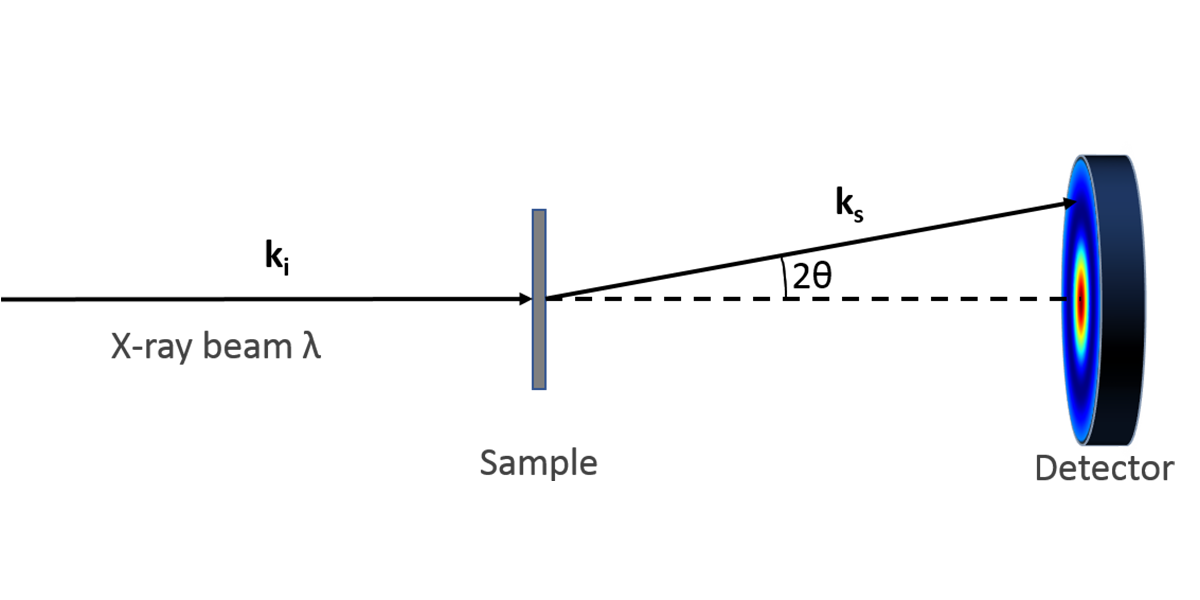
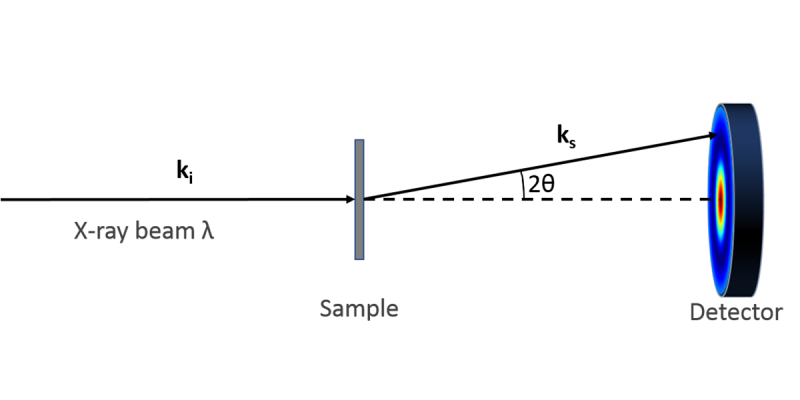
In a typical experiment, a highly collimated beam of monochromatic x-rays is transmitted through a sample on the order of 1 mm in thickness. The scattered x-rays are collected on a 2-dimensional area detector azimuthally in 360 degrees at a continuous range of scattering angles which deviate from the direct, transmitted beam. The scattering angle, which we define by convention as 2θ, defines a “probe length” expressed as D =2π/q, where q = 4πsin(θ)/λ, in which λ is the wavelength of the x-rays (typically 0.154 nm for the Cu x-ray source).
In the most common manifestation of a large molecular assembly (particle, lamellae, etc.), a peak in the scattering intensity as a function of q indicates dominant size at the corresponding D = 2π/q. Further analysis is used to refine the structure based on modeling the electron density variations in the material under study.
Advantages of SAXS
SAXS is a primary characterization tool that can provide structural information describing how large molecules are shaped and organize in any sample.
SAXS is accessible to the home laboratory, and does not require any extensive sample preparation, since the scattering contrast is determined by the inherent electron density differences native to the sample under study.
The only comparable tool is the neutron analog SANS (Small Angle Neutron Scattering), but this obviously requires a source of neutrons from a reactor or spallation facility, with the added burden/benefit of isotopic labelling to maximize neutron scattering contrast.
Other approaches by direct imaging are possible with electron microscopy and scanning probe microscopies, but these are limited to thin or topographic samples, respectively, and the results are non-statistical sample representation.
Dynamic and Static Light Scattering (DLS and SLS) are used in solution studies, but the results are model dependent and optical wavelengths will not provide the level of structural resolution for anything smaller ca. 0.5 µm.