Nanoparticles are small particles, with at least one dimension between 1 and 100 nanometers. Their high surface-to-volume ratio provides them with unique properties which makes them attractive for use in highly technological as well as more trivial applications, for example in construction industry. Due to limited purification options, control over size and shape during production are of utmost importance. X-ray scattering measurements can provide unparalleled access to these parameters and in addition, give insight into the mechanism of formation.
Nanoparticles are characterized by the fact that they have at least one dimension in the nanometer range, i.e. between 1 and 100 nanometers. This means that they can have any shape, and they can consist of a large range of materials. Some common examples are metals, silica, carbon, and polymers.
The properties of nanoparticles can be very different from those of the bulk material they are made from. They have a very high surface-to-volume ratio which means that the surface of the particle constitutes a significant part of the total material and this dominates the properties, resulting in stronger interactions with the environment. Their small size also influences magnetic and ferromagnetic properties, causes quantum mechanical behavior, and lastly, ordered packing of nanoparticles gives the materials new properties.
These new properties lay the basics for interesting applications. The large surface area enhances the interactions with solvent or matrix. This means that nanoparticle suspensions can be stabile despite the particle having a higher density than the solvent. In stabile suspensions, the particles are evenly distributed across the medium. This is important, for example, when nanoparticles are added to epoxy-resins.
Epoxy resins are widely used in the construction-industry as adhesives, sealants, paints, plastics and coatings.1 One big drawback of epoxies is that they are often brittle, a problem that is effectively remedied by the addition of metallic nanoparticles. Moreover, the addition of metallic nanoparticles results in better bonding and improved thermal and electrical conductivity. Which is a clear benefit for the automobile and aerospace industries.
To address the physical properties that give rise to the extraordinary functionality of these nanoscale materials, it is essential to obtain particles of a controlled size and shape, and of very low dispersity. Because the purification of nanoparticles is largely limited to washing, fractionation and separation and ultracentrifugation2, understanding of, and control over the synthesis is paramount. In addition, insight in the wet chemistry is required to optimize the synthesis process in terms of scalability and energy consumption.
Consequently, any investigation method allowing to provide size and shape information in real time, in the liquid matrix, without perturbing the reaction kinetics is extremely valuable. Moreover, it will help to reveal new mechanisms of particle growth besides classical nucleation rules. Whereas microscopy techniques can yield information about these important properties of nanoparticles, this cannot be done in real-time because a single measurement does not yield a statistically relevant outcome.
In situ analysis of nanoparticle synthesis
For the in situ analysis of reaction mixtures, short measurement times are required to be able to sample the synthesis process adequately. Furthermore, the technique needs to be compatible with the concentration of the reagents and products and the measurement itself should not interfere with the reaction.
Small-Angle X-ray Scattering (SAXS) offers an excellent means to this end. It allows for statistically relevant characterization of size distributions3 and the measurements can be done in situ in relevant sample conditions, without the need to disturb the reaction by taking a sample4,5. SAXS experiments can be performed together with simultaneous Wide-Angle X-ray Scattering (WAXS) measurements to assess the crystalline state of the sample under investigation.
Over the years, the SAXS/WAXS technique has been used efficiently for the investigation of the formation and growth mechanisms of nanoparticles, under realistic synthesis conditions, most of the time in the past using synchrotron facilities. While shorter time scales or ultrafast experiments (less than a second) remain the exclusivity of synchrotron experiments, recently it has been possible to experimentally follow the precursor reactions and subsequent nucleation using laboratory SAXS/WAXS set-ups6. More recently, nucleation and growth of iron oxide nanoparticles have been studied during high temperature synthesis (~300°C) using such type of X-ray scattering instruments7.
In addition, the use of a low maintenance sealed tube X-ray source is also possible to monitor colloidal synthesis including at the nucleation stage8,9 taking advantage of advanced optical components, low scattering noise instruments including advanced hybrid pixel detectors.
The picture below shows an example of a setup that has been used by scientists from USP Sao Paolo in Brazil in the frame of a collaboration with scientists from Duisburg-Essen University in Germany and IPEN in Brazil to study the formation of metallic nanoparticles during wet-chemical reduction synthesis9. The reaction – the formation of silver nanoparticles from a mixture of silver nitrate with glucose and polyvinylpyrrolidone (PVP) in water – is carried out in a heated vessel under continuous stirring. The reaction mixture is pumped into the SAXS instrument using a peristaltic pump and the measurements are done in a thermalized capillary. The measurements are done for 60 seconds each resulting in an average over these 60 seconds.

The obtained scattering patterns are reduced to 1D curves (symbols in the figure 2a below) and fitted (lines in the figure 2a below) using a Monte Carlo optimization method. The fits of the full dataset indicate that the silver nanoparticles consist of two major populations: one with a diameter of ~3 nm, and one with a diameter ranging from 10 to 40 nm.
From this data, the volume fraction of both types of particles can be derived, as depicted in the figure 2b below. Here, it is clearly shown that the volume fraction of small nanoparticles dominates at the start of the synthesis. These particles continuously grow to larger particles, which dominate towards the end of the synthesis process. The mechanism of formation can be derived from the growth rates. Up until 51 minutes, this is dominated by coalescence, while the end of the process is dominated by Ostwald ripening.
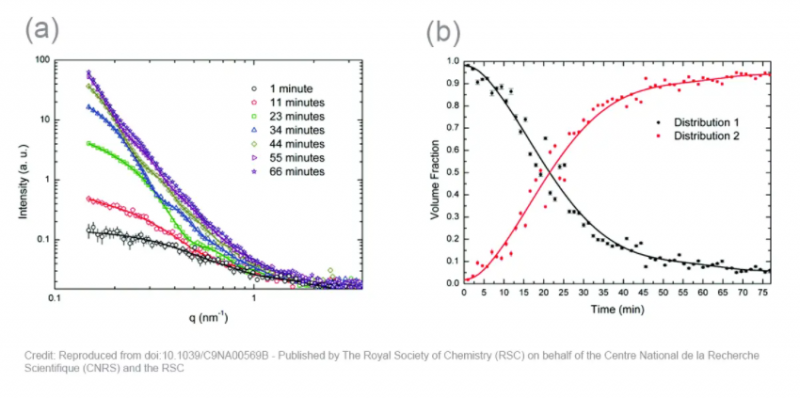
This example shows that in situ SAXS analysis of the formation of silver nanoparticles does not only offer detailed information of the size and shape of the formed particles, but also about the size distribution and the mechanism of formation. This enables control over the outcome of the synthesis by quenching the reaction.
The Xenocs Xeuss and the Nano-inXider are excellently suited for this type of measurements. The Xeuss is a high-performance SAXS beamline for laboratory use, designed to facilitate Ultra-Small-, Small- and Wide-Angle X-ray Scattering (USAXS, SAXS & WAXS) for soft matter and nanomaterial analysis. While the Nano-inXider helps accelerate research by combining superb performance with great user experience and a small footprint.
In Vietnam, please do not hesitate to contact us for more information and consultation for any of your applications with this beautiful product from #Xenocs.
References and Further Reading:
- https://blog.agchemigroup.eu/why-use-nanoparticles-as-a-raw-material-in-epoxy-resins/
- https://www.sigmaaldrich.com/technical-documents/articles/biology/nanoparticle-ultrafiltration.html
- Xenocs Application Note ”Size distribution of Nanoparticles: powders, dispersions and composites“.
- Li T, Senesi AJ, Lee B. Small Angle X-ray Scattering for Nanoparticle Research. Chem Rev. 2016;116(18):11128-11180. doi:10.1021/acs.chemrev.5b00690
- MourdikoudisS, Pallares RM, Thanh NTK. Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale. 2018;10(27):12871-12934. doi:1039/C8NR02278J
- Chen X,Schröder J, Hauschild S, Rosenfeldt S, Dulle M, Förster S. Simultaneous SAXS/WAXS/UV–Vis Study of the Nucleation and Growth of Nanoparticles: A Test of Classical Nucleation Theory. Langmuir. 2015;31(42):11678-11691. doi:1021/acs.langmuir.5b02759
- Leffler V, Ehlert S, Förster B,Dulle M, Förster S. Nanoparticle Heat-Up Synthesis: In Situ X-ray Diffraction and Extension from Classical to Nonclassical Nucleation and Growth Theory. ACS Nano. 2021;15(1):840-856. doi:1021/acsnano.0c07359
- ChemSAXS: study of chemical reactions inreal time https://www.youtube.com/channel/UC6kdn4epvHF4OZM7T-mLXJQ
- F. Garcia, P. R. A.,Prymak, O.,Grasmik, V., Pappert, K., Wlysses, W., Otubo, L., Epple, M. & P. Oliveira, C. L. An in situ SAXS investigation of the formation of silver nanoparticles and bimetallic silver–gold nanoparticles in controlled wet-chemical reduction synthesis. Nanoscale Adv. (2020). doi:10.1039/C9NA00569B